Research Goals
During development, precise control of gene expression allows the reproducible establishment of patterns, leading to the formation of organs at the right time and place. What are the mechanisms behind such precision?
The establishment of developmental patterns has been primarily studied at the transcriptional level. In comparison, the fate of these transcripts received little attention. Development of new methods enabling the study of translation of single mRNA molecules revealed a critical heterogeneity in translation. Our lab pioneered the deployment of such methods in Drosophila embryos, leading to the discovery of translation factories and unmasking intragenic translation heterogeneity, demonstrating a novel layer of variation in gene expression. Thus, to decipher the mechanisms dictating precision in gene expression, it is essential to simultaneously consider both layers of regulation constituted by transcription and translation as well as their potential coupling.
We propose to bridge the gap between these two pillars of the central dogma to obtain an integrated multiscale view of gene expression control. We use quantitative imaging methods to simultaneously monitor mRNA and protein in living Drosophila embryos with unprecedented spatio-temporal resolution, combined with theoretical models to formulate predictions that will be tested by genetic/optogenetic manipulations.
From gene expression to tissue patterning
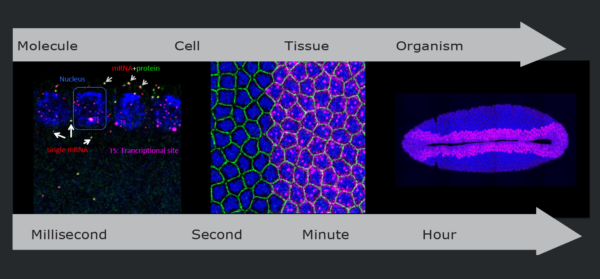
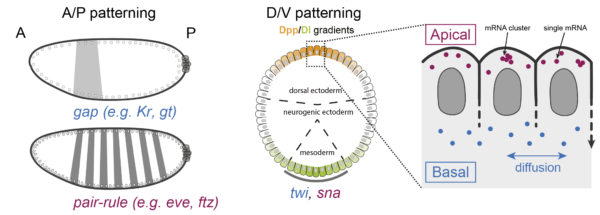
Approach
We mainly study early Drosophila development: the first 4 hours of embryogenesis.
The approach is highly integrative with techniques ranging from classical genetics and molecular biology to whole-genome profiling and state of the art live imaging microscopy. Image quantification and mathematical modeling are important aspects of our research.
Imaging transcription in living embryos
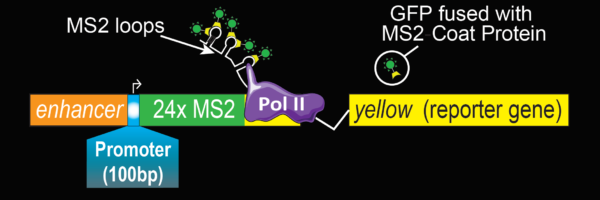
Principle of the MS2 approach
Imaging translation in living embryos
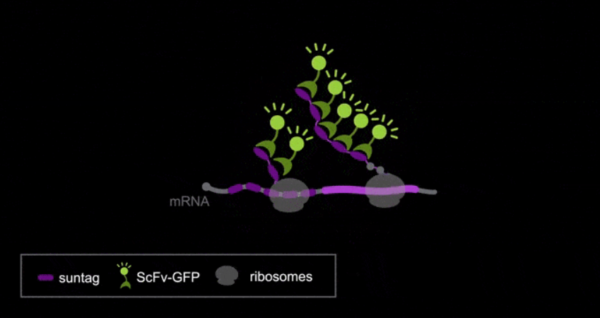
Principle of the SunTag approach

